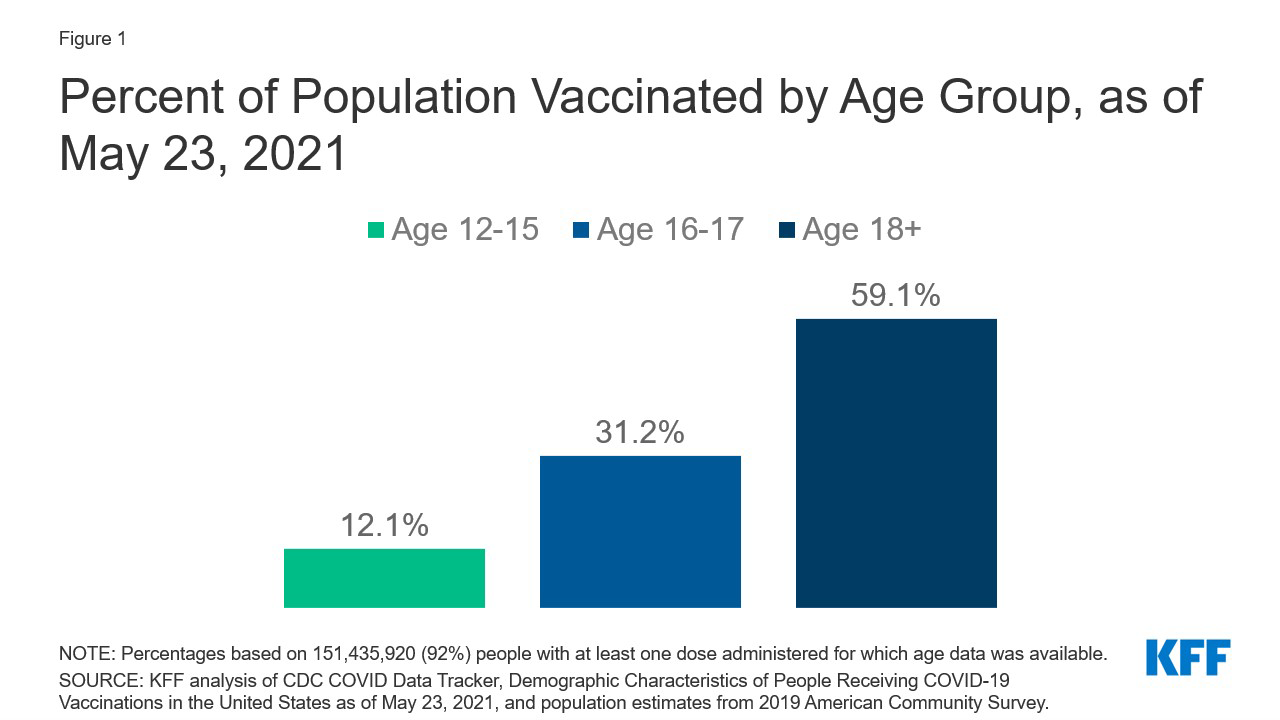
With the recent authorization of Pfizer’s COVID-19 vaccine for adolescents, ages 12-15, a group that totals almost 17 million, the next phase of the U.S. vaccination effort has begun. Authorization for even younger children is expected as early as the fall, and even before this point, Pfizer’s vaccine had been authorized for 16-17 year-olds. This has focused attention on the role of parents and parental consent for vaccination, especially since most parents are not yet ready to get their child vaccinated. In our most recent survey, fielded just before the FDA’s authorization for adolescents, 3 in 10 parents said they would do so right away, with most instead wanting to wait and see or saying they would not get their child vaccinated at all or would do so only if required for school. As of May 23, more than 2.0 million 12-15 year-olds and 2.5 million 16-17 year-olds had received at least one vaccine dose, approximately 12% and 31% of adolescents in each age group, respectively (Figure 1). To better understand the landscape of parental consent laws, we assessed which states have such laws, for what ages, and where exceptions for COVID-19 vaccination have been made.

Figure 1: Percent of Population Vaccinated by Age Group, as of May 23, 2021
Overall, we find that most states require parental consent at this point, though the landscape may be shifting slightly as more jurisdictions seek to encourage vaccination of young people. Specific findings are as follows:
As we find here, parents, and parental consent laws, will play a critical role in the COVID-19 vaccination effort to reach children in the U.S., particularly as authorization moves to even younger ages. Most states have already required parental consent for 16-17 year-olds, who have been eligible for vaccination since the nation’s vaccine effort began. There are only a handful of states where minors under the age of 16 can self-consent. As such, reaching parents with information about COVID-19 vaccination for children will be the driving factor for increasing vaccine coverage for young people. At the same time, the legal landscape may be shifting slightly. Similar to laws in a handful of states allowing minors to self-consent for certain other services (e.g., HIV and STI testing and treatment), some cities and states are moving to allow self-consent for minors for the COVID-19 vaccination specifically, in an effort to increase vaccinations among young people.